Security, performance, and reliability are woven into every aspect of Propel.
.png)




.png)
.png)
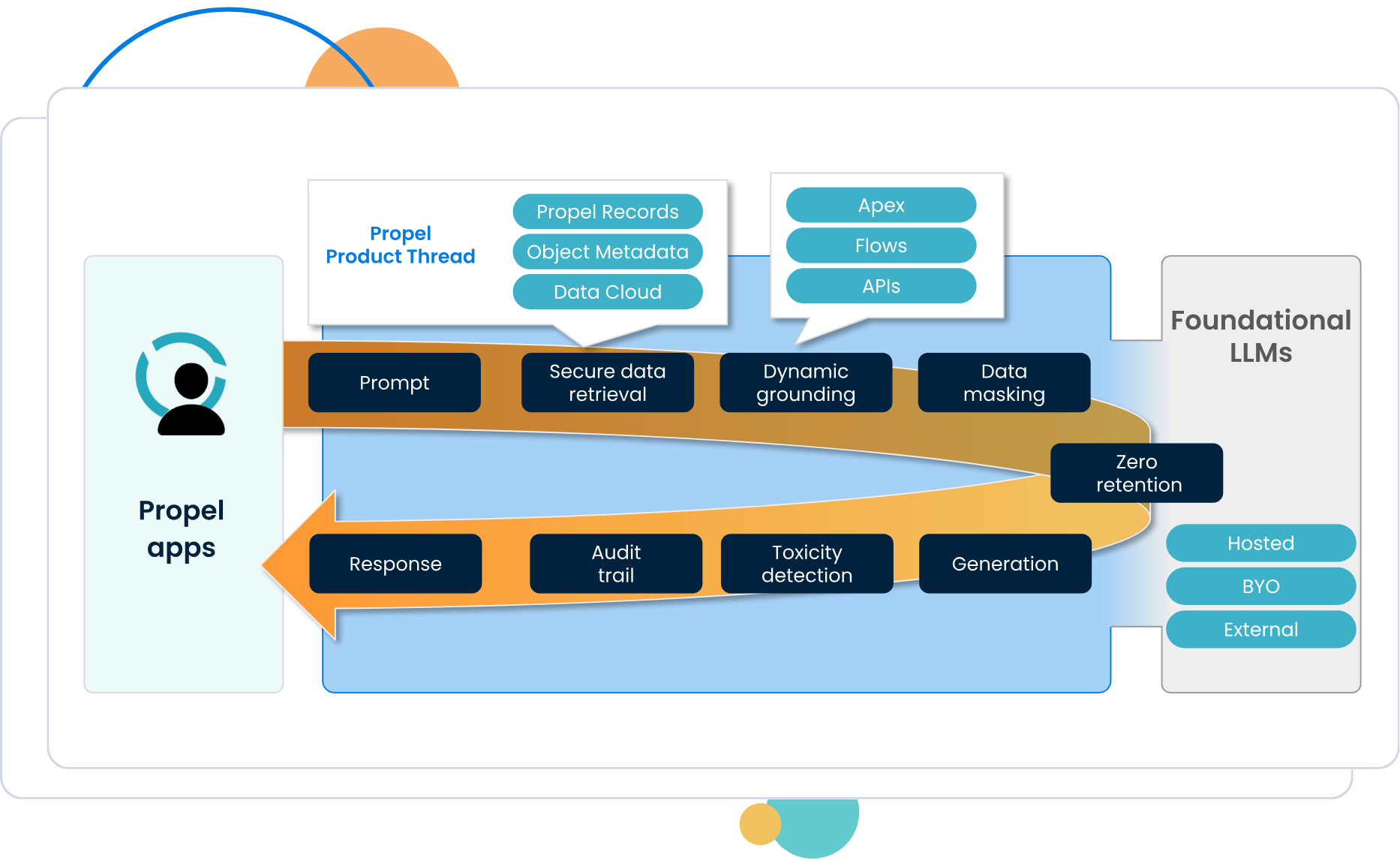
Your data is gold. Propel keeps it private and secure. Using the Agentforce Trust Layer, your data is never used to train LLMs for others.
Topics and actions follow user permissions and instructions to gather relevant data.
Dynamic grounding for LLMs ensures accuracy and prevents hallucinations.
Data masking anonymizes sensitive data to comply with privacy requirements. (More on data masking).
Large language model (LLM) is the internal or external AI specified to understand the prompt and generate the response, such as ChatGPT, Google Gemini, or Claude. (More on data masking).
Zero retention erases prompts and outputs, without storing or training AI models.
Toxicity detection flags toxic content, such as aggressive language. (More on toxicity scoring).
AI auditing ensures operating in line with regulatory and organizational frameworks. (More on audit trail with Data Cloud).
We’ve got you covered. Find answers to common questions related to security.








A link to our Product Tour has been sent to you via email.
We’ve received your contact request. A member of our team will be reaching out shortly to set up a time to talk via email.